Background: Anti-factor VIII (FVIII) inhibitory antibody development (inhibitors) occurs in up to 13% of patients with non-severe hemophilia A (HA) and is known to be associated with receipt of intensive FVIII treatment (Eckhardt et al. 2013) and F8 mutations such as p.Arg612Cys. Inhibitor development is a T-cell dependent process requiring presentation of a novel peptide by HLA-class II on the surface of antigen presenting cells. Computer algorithms can be used to model the binding affinity of a peptide for a specific HLA type (Zhang et al. 2012). The strength of association between computer-modeled HLA-DRB1 and mutation-specific FVIII peptide binding and inhibitor development in non-severe HA with known F8 and HLA-DRB1 genotype is unknown.
Methods: In a cohort of non-severe HA (FVIII 1-40%) collected as part of a case-control study (Kempton et al. 2010), subjects with a missense mutation who had consented to participate in a repository for future research were evaluated. Case subjects had an inhibitor titer > 1.0 BU/ml on two occasions or on one occasion with subsequent immune tolerance induction. Control subjects had prior FVIII exposure and no history of inhibitor (< 0.6 BU/ml). F8 genotyping was performed at the Hemostasis Laboratory Branch at the Centers for Disease Control and Prevention. HLA-DRB1 type was determined by Next Generation Sequencing performed at HistoGenetics Laboratory (Ossining, NY). Using the subject's HLA-DRB1 type and a series of fifteen 15-mer peptides that contained the subject's missense mutation in each position of the 15-mer peptide, the MHC-II Binding Predictions Tool (iedb.org) was used to predict the binding affinity of each of the fifteen 15-mer peptides to both of the HLA-DRB1 alleles (30 potential binding opportunities for each subject). Binding affinity to the wild-type (treatment [t] FVIII) was also estimated. A peptide was considered as binding if the predicted 50% inhibitory concentration was < 1000 nmol/L. Each peptide-HLA-DRB1 interaction was categorized into 1 of 4 groups (A-D) (Shepherd et al 2015). Non-novel peptides were present if both endogenous (e) FVIII and tFVIII peptides were non-binding (group A) or if both peptides were binding but tFVIII did not alter the binding core (group C). Novel peptides were present if the eFVIII sequence was a non-binder and the tFVIII was a binder (group B) or if both tFVIII and eFVIII bound but tFVIII altered the binding core (group D). The association between inhibitor development and the number of 15-mer peptides that could present a novel peptide (group B/D; maximum = 30) was evaluated in simple and multivariable logistic regression models. Age, family history of inhibitor, and intensive FVIII treatment were included in an adjusted model based on prior literature. Other variables were evaluated and included if found to be a confounder.
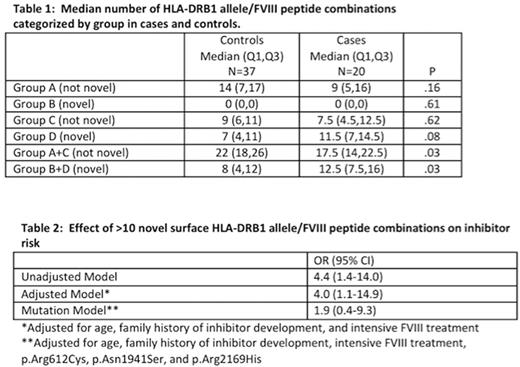
Results: Fifty-seven subjects (case n=20 and control n=37, 56% of the original study sample) were included in this analysis. The median age (case 37 years vs control 27 years) and baseline FVIII (case 8.0 IU/dl vs control 5.5 IU/dl) were similar between groups. Cases and controls were also similar with respect to race, ethnicity, prior FVIII exposure, family history of inhibitor, FVIII product class used (recombinant vs. plasma-derived), and being HIV or HCV antibody positive. F8 mutations found in more than 1 case included p.Arg612Cys (case=6; control=1), p.Asn1941Ser (case=5; control=1), and p.Arg2169His (case=3; control=1). HLA alleles found both in more than 10% of cases and more frequently in cases than controls included HLA-DRB1*15:01 (case =7 [18%], control =7 [9%], OR 2.3 [95% CI 0.5-10.8]), HLA-DRB1*13:02 (case=5 [13%], control=4 [5%], OR 2.8 [95% CI 0.5-16.4]); HLA-DRB1*11:01 (case=4 [10%], control = 3 [4%], OR 3.0 [95% CI 0.4-20.2]). The median number of novel HLA-DRB1 allele/peptide combinations among cases and controls is shown in Table 1. The association of having > 10 novel HLA-DRB1 allele/peptide combinations with inhibitor development is shown in Table 2.
Conclusions: Having more than 10 FVIII peptides (>1/3 of the potential FVIII peptides) that are able to bind HLA-DRB1 and present a novel surface is associated with inhibitor development independent of age, family history of inhibitor, and intensive FVIII treatment. The presentation of novel HLA-DRBI peptide combinations appears to be an intermediary between specific high-risk F8 mutations and inhibitor development.
Kempton: Genentech: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.